Entropy & Clausius Inequality
Entropy is a property
- A measure of the degree of microscopic disorder in the system
- Entropy can be transferred by heat but not by work
- Entropy is not conserved
- Entropy can be generated/created but not destroyed
- Total entropy for isolated systems (system + surroundings) increases for all real processes
Clausius Inequality
Cyclic integral of 𝛿𝑄/𝑇 is always less than or equal to zero for all cycles regardless of reversible, irreversible cycles
- Unit for T must be absolute temperature
For reversible cycles
Example: Carnot Heat Engine
For irreversible cycles
Example: Any other heat engine
Generally, cyclic integrals of any property is zero
Hence Entropy is a property:
Entropy of Pure Substances
Entropy data for pure substances (including real gases) can be obtained from property tables
Obtaining Entropy change (using property table)
The difference between the entropy of the initial state vs the finals state ** Interpolation may be needed
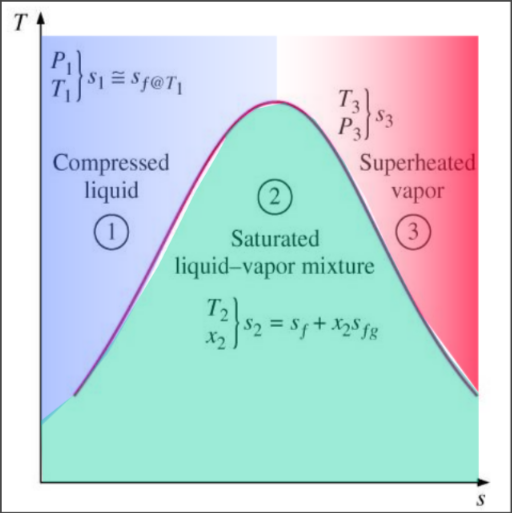
Property Diagrams Involving Entropy
2nd Law analysis: T-s & h-s diagrams
Analysing T-s Diagrams
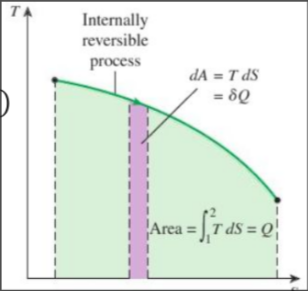
Differential form of entropy:
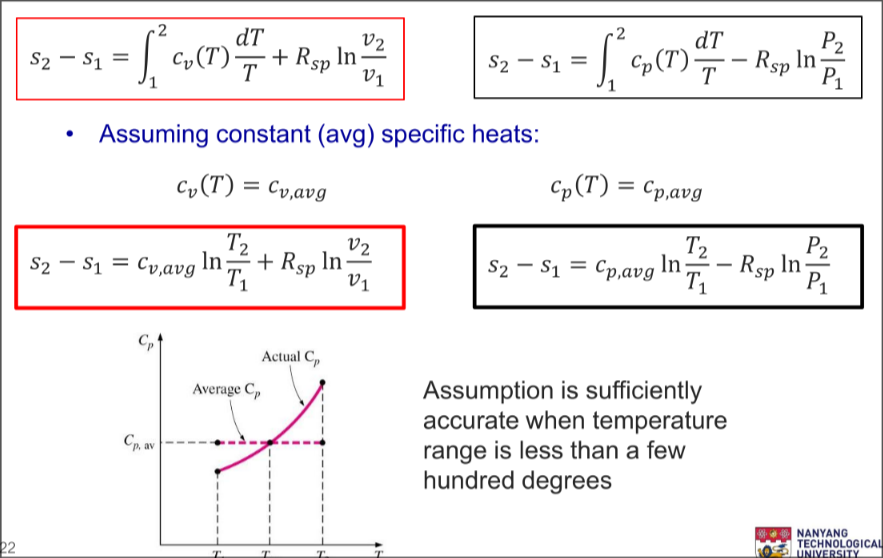
Integrating entropy:
Tip
Area under the process curve in the T-s diagram represents the heat transfer in an internally reversible process!
Special conditions
Internally reversible isothermal process
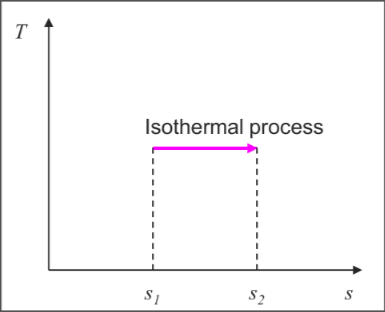
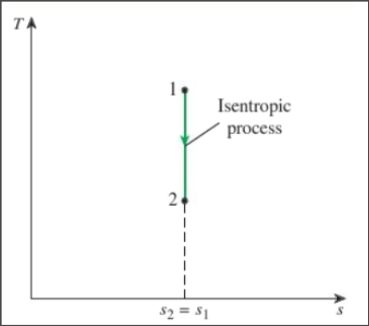
Isentropic process
Constant entropy — vertical line segment
Tds Relations (Entropy change without Property table)
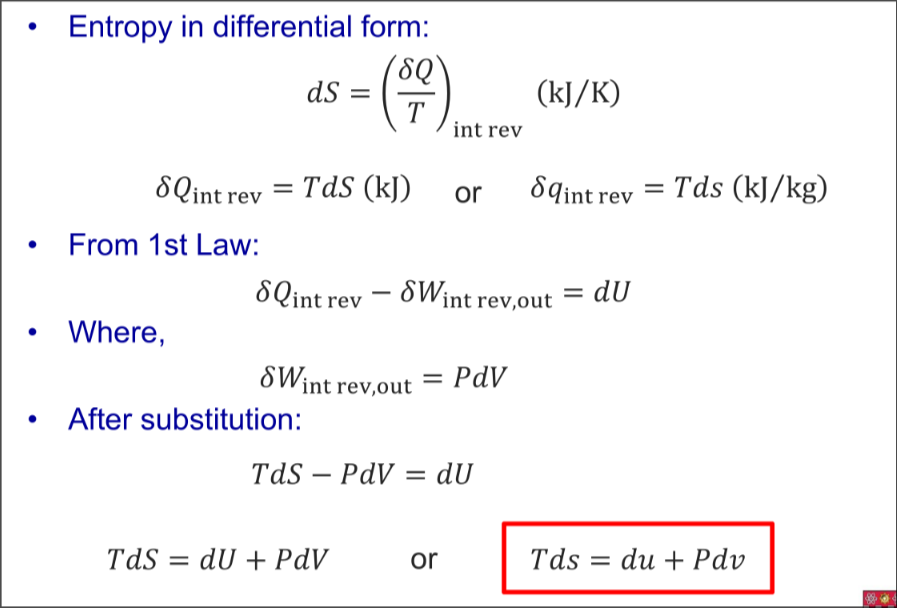
1st Tds Equation (Gibbs Equation)
Expand for Derivation
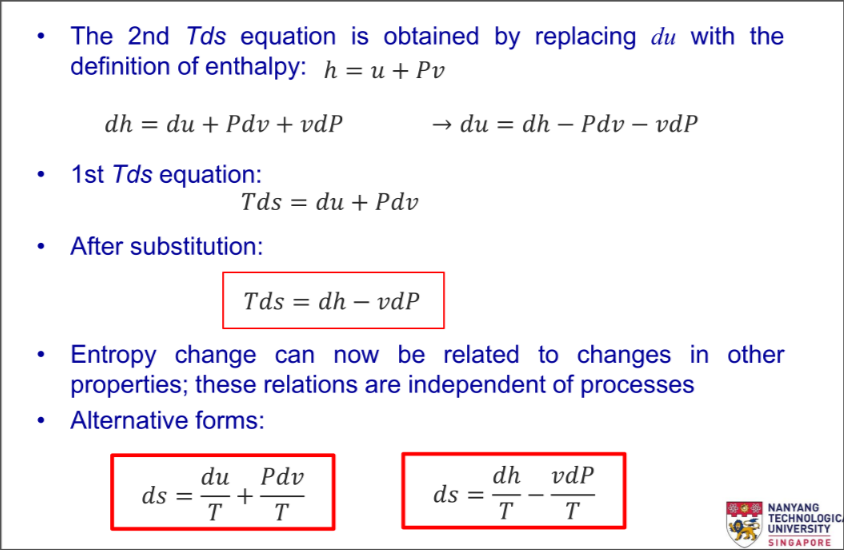
2nd Tds Equation
Alternative forms:
Expand for Derivation
Entropy Change of Liquids & Solids
Liquids and solids can be approximated as incompressible substances, hence:
- 𝑑𝑣 ≅ 0
Thus, the formula:
- Entropy change for liquids and solids are only dependent on temperature and independent of pressure
For isentropic
Entropy Change of Ideal Gases
Ideal gas equations
where:
Thus, the formulas:
Note:
Expand for Derivation
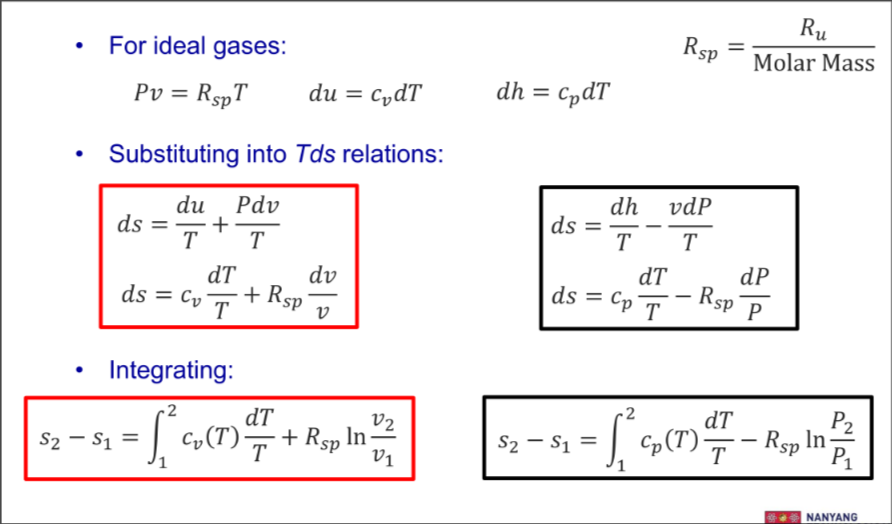
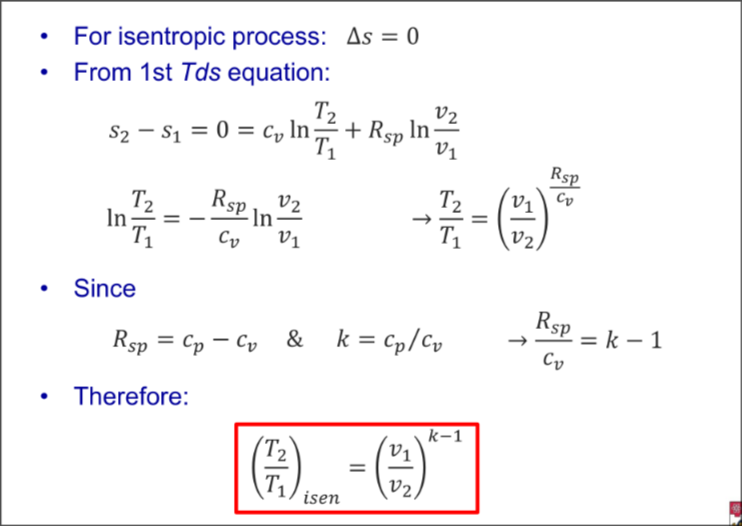
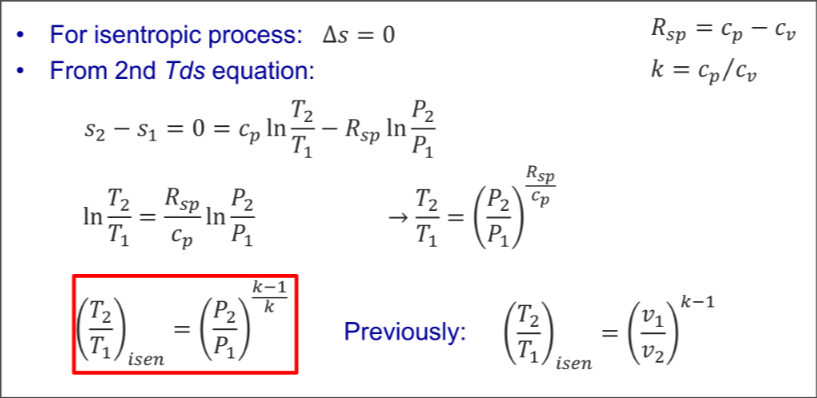
For isentropic
From 1st Tds equation
Expand for Derivation
From 2nd Tds equation
Expand for Derivation
Combined
Entropy Change for Reversible Processes
Internally reversible isothermal heat transfer
- Where T0 is the constant temperature (K), Q is the heat transfer for the internally reversible process
- Commonly used to determine the entropy change of thermal energy reservoirs that supply/absorb heat indefinitely at constant temperature
Entropy Change for Irreversible Processes
For irreversible process, the entropy change can be determined along an imaginary internally reversible path
Entropy Change
Additional entropy change due to presence of irreversibilities
- Always positive (irreversible) or zero (reversible)
- Not a property; value is process dependent
\text{Entropy change of} &= \text{Entropy transfer} + \text{Entropy generation} \\ \text{a closed system} &\quad \text{due to heat transfer} \quad \text{due to irreversibilities} \end{align}$$ ## Differential form $$ dS \geq \frac{\delta Q}{T}$$ > [!NOTE]- Expand for Derivation > > ![[Pasted image 20250916132408.png]] --- # Entropy Change for Systems $$\begin{aligned} &\boxed{\Delta S_{system} = S_{in} - S_{out} + S_{gen}} \\[1em] \text{Change in total} &= \text{Total} - \text{Total} + \text{Total} \\ \text{entropy of the} &\quad \text{entropy} \quad \text{entropy} \quad \text{entropy} \\ \text{system} &\quad \text{entering} \quad \text{leaving} \quad \text{generated} \end{aligned}$$ ### Entropy Changes Entropy change is zero in steady flow devices during steady operation (turbines, compressors, pumps, nozzles, diffusers, heat exchangers etc.) $$\Delta S_{system} = S_{final} - S_{initial} = 0$$ ### Entropy Transfer For closed systems, entropy is transferred only by heat $$S_{in} - S_{out} = \int_1^2 \frac{\delta Q}{T} \cong \sum \frac{Q_k}{T_k}$$ $$Q_{in} \xrightarrow{\text{}} \boxed{\begin{array}{c} \text{Closed} \\ \text{System} \end{array}} \xrightarrow{\text{}} Q_{out}$$ - $Q_k$ = heat transfer - $T_k$ = boundary temperature through which heat is transferred ### Entropy Generation A measure of entropy created by irreversibilities during a process (friction, heat transfer via finite temperature difference, mixing etc.) $$S_{gen} \begin{cases} > 0 & \text{irreversible process} \\ = 0 & \text{reversible process} \\ < 0 & \text{impossible process} \end{cases}$$ ## Entropy Change — Closed Systems $$\Delta S_{closed} = S_2 - S_1 = \sum \frac{Q_k}{T_k} + S_{gen}$$ - $Q_k$ = heat transfer - $T_k$ = boundary temperature through which heat is transferred ### Special case — adiabatic system Change in entropy is only due to entropy generation within the system boundaries $$\Delta S_{closed} = S_2 - S_1 = \cancel{\sum \frac{Q_k}{T_k}} + S_{gen}$$ $$\Delta S_{adiabatic\ system} = S_{gen} > 0$$ - Since entropy generation for irreversible processes can never be less than zero, the entropy in an adiabatic closed system always increases ### Special case — isentropic process $$ \Delta s =0 \quad \rightarrow \quad \Delta S_{isen} = S_2 - S_1 = \cancel{\sum \frac{Q_k}{T_k}} + \cancel{S_{gen}} $$ - *Isentropic = Reversible Adiabatic Process* ### Special case - Isolated systems No mass, heat or work transfer for an isolated system $$\Delta S_{isolated} = S_2 - S_1 = \cancel{\sum \frac{Q_k}{T_k}} + S_{gen}$$ $$\Delta S_{isolated} = S_{gen} > 0$$ An isolated systems may consist of any number of subsystems; total entropy = sum of its parts $$\Delta S_{total} = \sum_{i=1}^{N} \Delta S_i > 0$$ --- # Entropy Change for Flow Control Volumes $$(S_2 - S_1)_{CV} = \sum m_i s_i - \sum m_e s_e + \sum \frac{Q_k}{T_k} + S_{gen}$$ $$ \text{Change in Entropy} = \text{Net entropy transfer by heat and mass flow}+\text{Entropy generation}$$ Rate Form: $$\dot{S}_{CV} = \sum \dot{m}_i s_i - \sum \dot{m}_e s_e + \sum \frac{\dot{Q}_k}{T_k} + \dot{S}_{gen}$$ ![[Pasted image 20250916213327.png|400]] ## Steady State, Steady Flow situation $$\boxed{\sum_k \frac{\dot{Q}_k}{T_k} + \dot{S}_{gen} + \sum_i \dot{m}_i s_i - \sum_e \dot{m}_e s_e = 0}$$ - rate of entropy change is 0 > [!NOTE] Recap: Energy balance for heat exchanger (flow control volume) > > $$\sum \dot{m}_i h_i - \sum \dot{m}_e h_e + \dot{Q} - \dot{W} = 0$$ ## Single Stream, Steady Flow $$\dot{m}_i = \dot{m}_e = \dot{m}$$ $$\dot{m}(s_e - s_i) = \sum \frac{\dot{Q}_k}{T_k} + \dot{S}_{gen}$$ ### Adiabatic - Entropy of fluid will increase as it flows through an adiabatic device $$\dot{m}(s_e - s_i) = \cancel{\sum {\frac{\dot{Q}_k}{T_k}}} + \dot{S}_{gen}$$ $$\dot{S}_{gen} \geq 0 \quad \rightarrow s_e \geq s_i$$ ### Adiabatic & Reversible → Isentropic Flow $$\dot{m}(s_e - s_i) = \cancel{\sum \frac{\dot{Q}_k}{T_k}} + \cancel{\dot{S}_{gen}}$$ $$\rightarrow s_e = s_i$$ --- # Reversible Steady Flow Work When $\Delta ke \approx 0$ & $\Delta pe \approx 0$: $$w_{rev} = -\int_i^e v \, dP$$ - W is positive → work done by fluid > [!NOTE]- Expand for Derivation > > ![[Pasted image 20250916231137.png]] ## Special case — Incompressible fluid When v is constant & $\Delta ke \approx 0$ & $\Delta pe \approx 0$: $$w_{rev} = -v(P_2 - P_1)$$ ## Special case — Fluid does no work and incompressible (eg. pipes) $$0 = -v(P_2 - P_1) - \frac{V_2^2 - V_1^2}{2} - g(z_2 - z_1) \quad \text{Bernoulli Equation}$$ - V represents fluid velocity as opposed to specific volume --- # Polytropic Work in Steady Flow Devices $$w_{poly} = -\int_1^2 v \, dP = -\int_1^2 \left(\frac{C}{P}\right)^{1/n} dP$$ $$= -\frac{n}{n-1}(P_2 v_2 - P_1 v_1)$$ Polytropic means:
Pv^n = \text{const.}$$
For Ideal Gas
Isentropic for Ideal Gas (n=k)
Due to the rule that for isentropic
Isothermal (n=1)
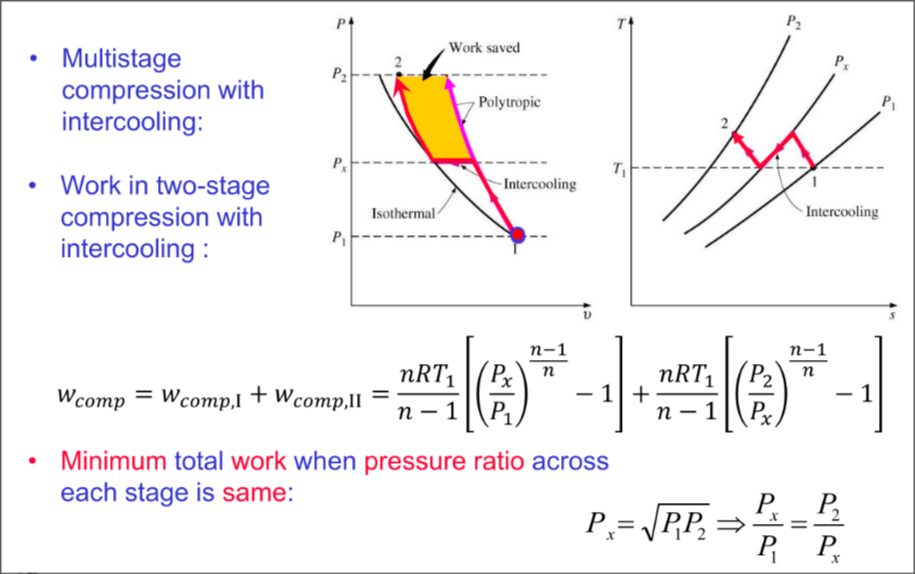
Min Compressor Work
Steady State Control Volume Devices
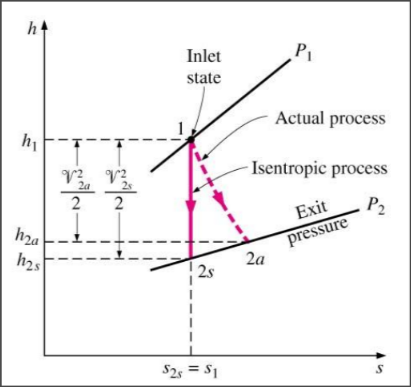
SSCV devices are near adiabatic and work best when irreversibilities are minimized, making isentropic processes the ideal models for these devices.
Isentropic efficiency
The measure of the deviation of the actual (adiabatic) process from the idealized one
Isentropic Efficiency for Turbines
- 90% for large turbines
- 70% for small turbines
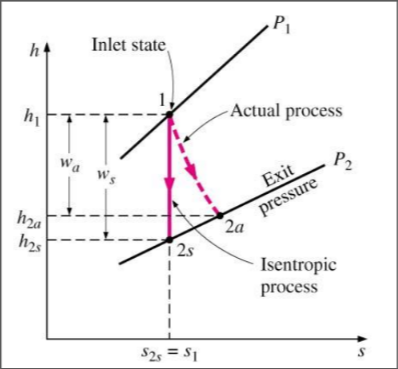
Isentropic Efficiency for Compressors
where,
- power consumed in isentropic is lower than actual
- 75-85% for well designed
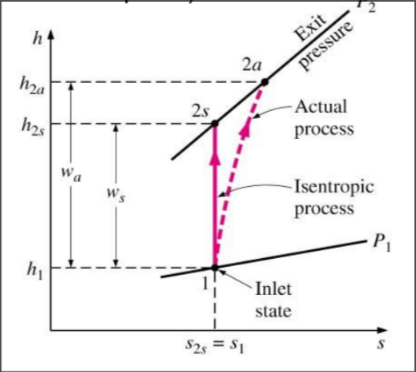
Isentropic efficiency for Pumps
Isentropic Efficiency for Nozzles
- V represents fluid velocity
- Typical