Recap on Prerequisites
The 2nd Law of thermodynamics is all about the efficiency of heat transfer processes
Thermal Energy Reservoirs
Hypothetical body with a relatively large thermal energy capacity (mass x specific heat) that can absorb/supply finite amounts of heat without undergoing any change in temperature.
Source - supplies heat energy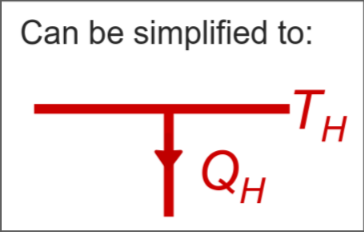
Sink - absorbs heat energy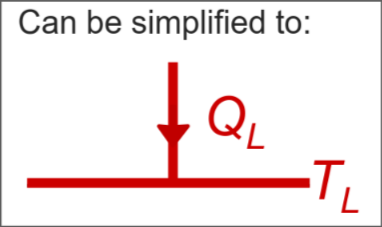
Heat Engines
Converts heat to work
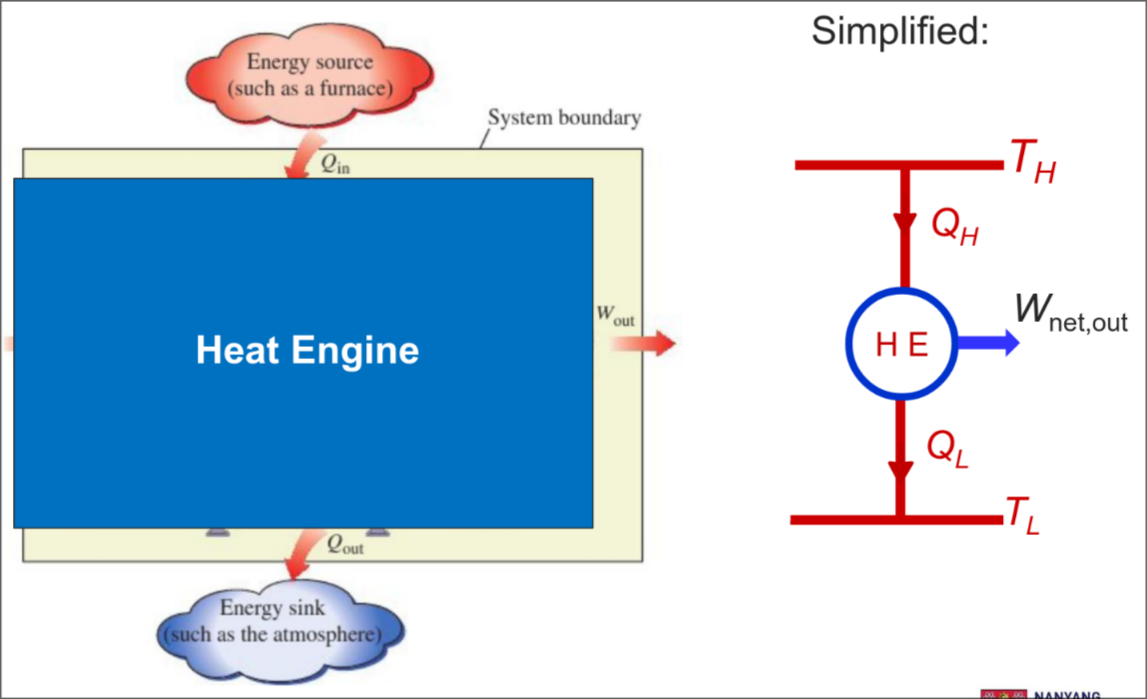
Energy Balance
From first law:
The energy balance for heat engines is:
Thermal Efficiency
So for Heat Engines:
Kelvin-Planck Statement
*It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce a net amount of work.
- Even theoretically perfect heat engines do not have an efficiency of 100%
Reverse Heat Engines
Work is added to induce heat transfer from low temperatures to high temperatures
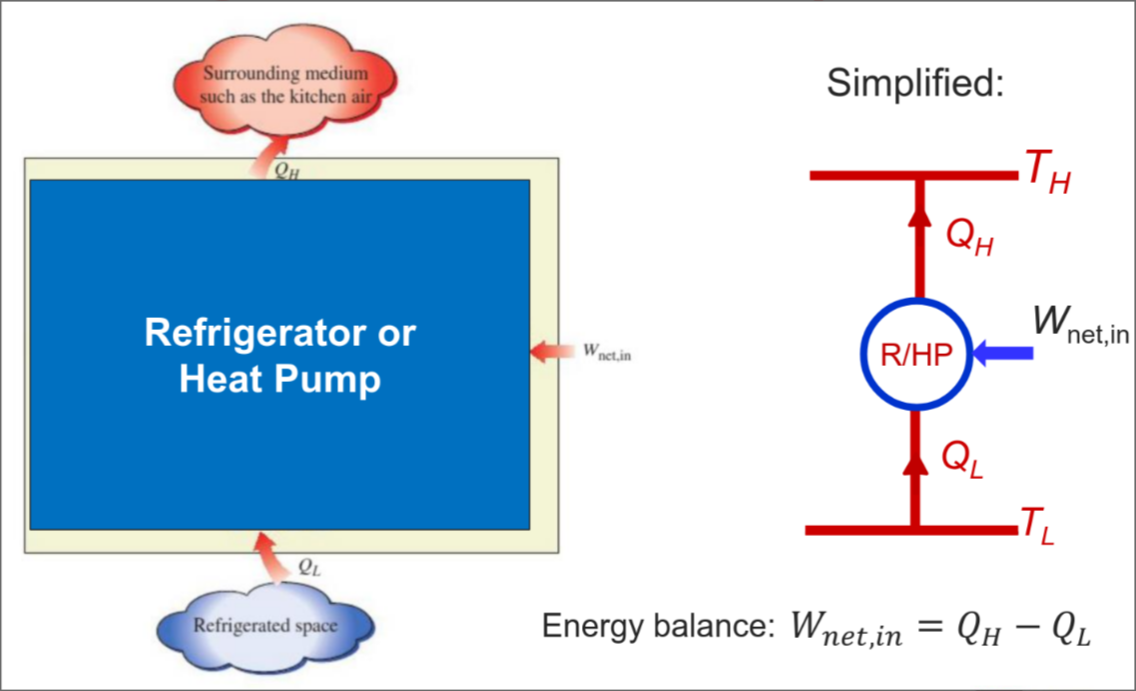
- E.g. Refrigerator / Heat pumps
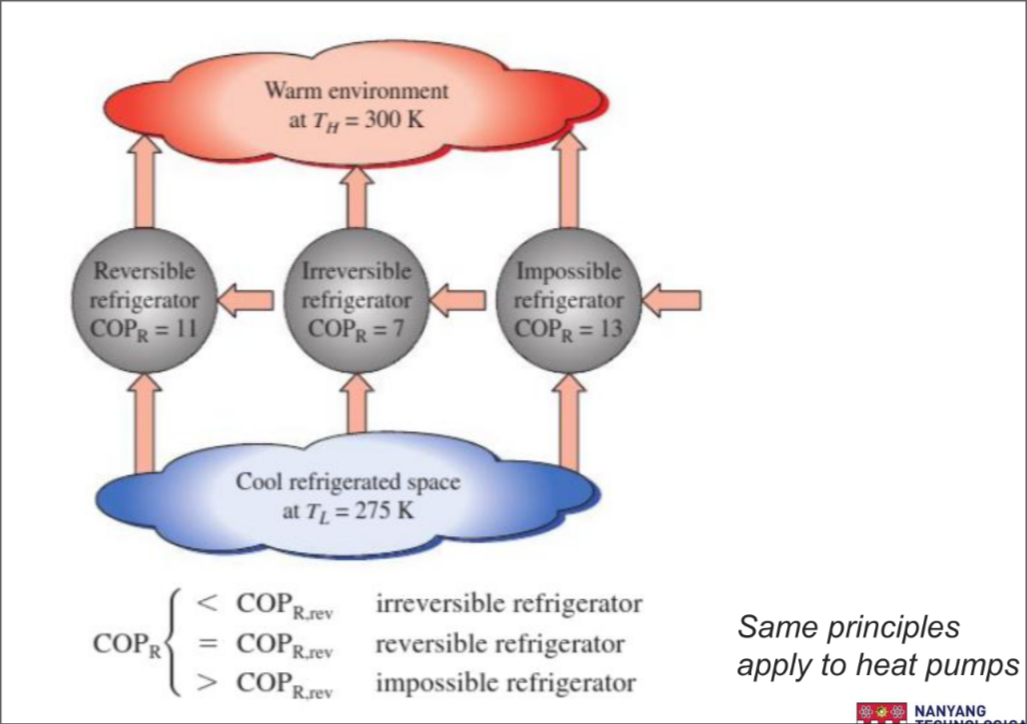
Coefficient of performance
For Refrigerator: Function - to cool refrigerated space
For Heat Pump: Function - to warm heated space
Clausius Statement
It is impossible to construct a device that operates in a cycle and produces no effect other than the transfer of heat from a lower-temperature body to a higher-temperature body.
Perpetual Motion Machine
A device that contradicts either the 1st law or 2nd law of thermodynamics
Recap
1st Law - energy cannot be created or destroyed, only transformed from one form to another
2nd Law - the entropy, or disorder, of a closed system never decreases
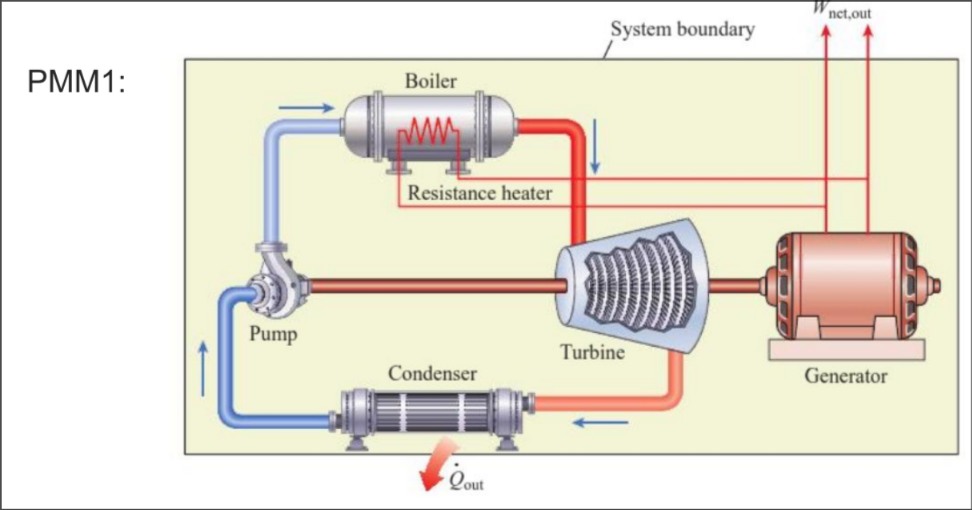
PMM1: Infinite output with no inputs
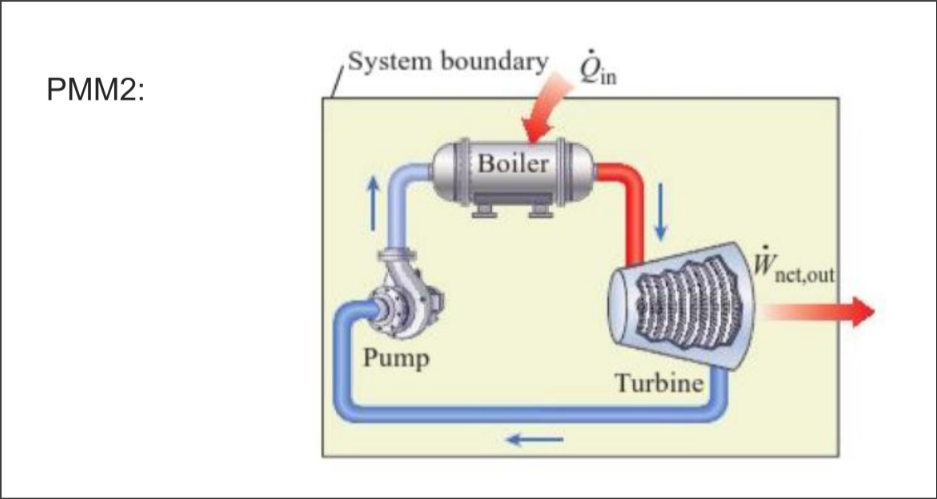
PMM2: decreasing entropy
Reversible & Irreversible processes
Reversible process - Can be reversed without leaving traces in the surrounding
- E.g frictionless pendulum
Irreversible process - opposite - Most processes in real life
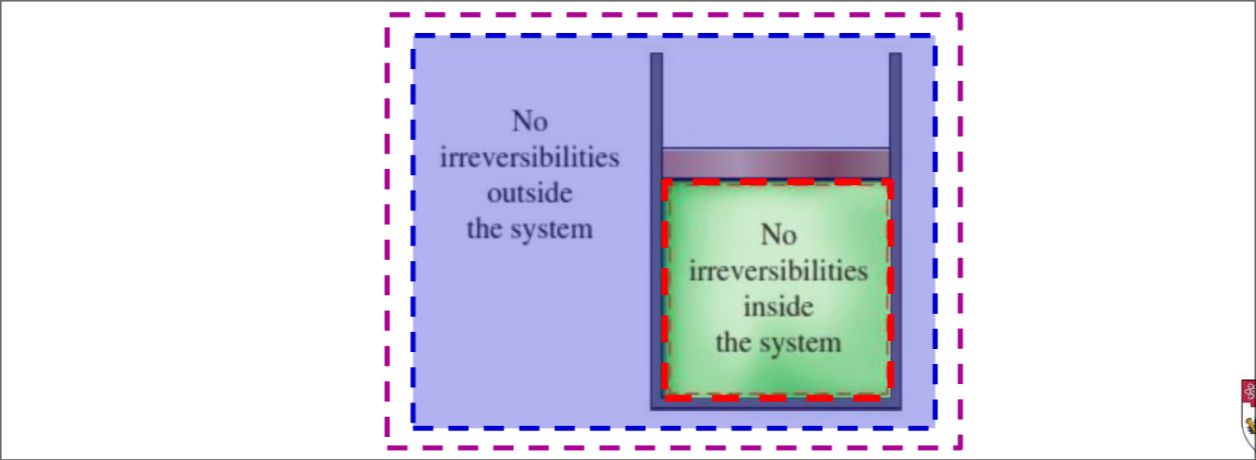
Internally reversible - no irreversibilities occur within system boundaries (red box)
Externally reversible - no irreversibilities occur outside system boundaries (blue box)
Totally reversible - internally + externally reversible (magenta box)
Carnot cycle
A theoretical cycle that sets the limits for heat engines, refrigerator and heat pumps
Recap
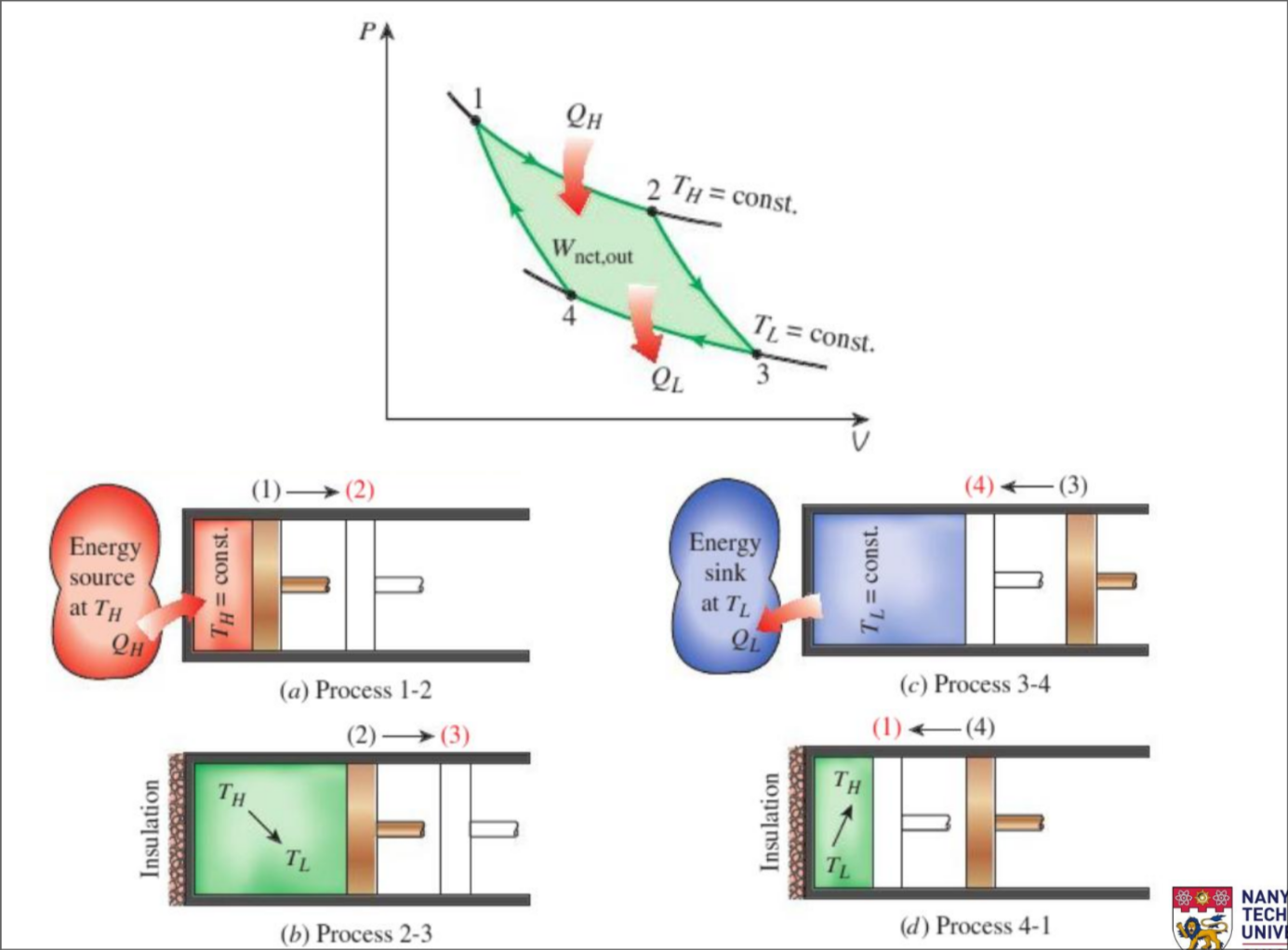
(a) Reversible Isothermal Expansion (1-2, T_H = constant)
- Gas expands at constant temp while absorbing heat from energy source
(b) Reversible Adiabatic Expansion (2-3, T_H drops to T_L)
- Gas does work on surrounding and expands while its temp drops
(c) Reversible Isothermal Compression (3-4, T_L constant)
- Gas compression at constant temp while losing heat to energy sink
(d) Reversible Adiabatic Compression (4-1, T_L rises to T_H)
- Work done on gas to compress it and its temp rises
The reversed carnot cycle
The carnot cycle is a reversible cycle that nets the “Carnot refrigeration cycle”
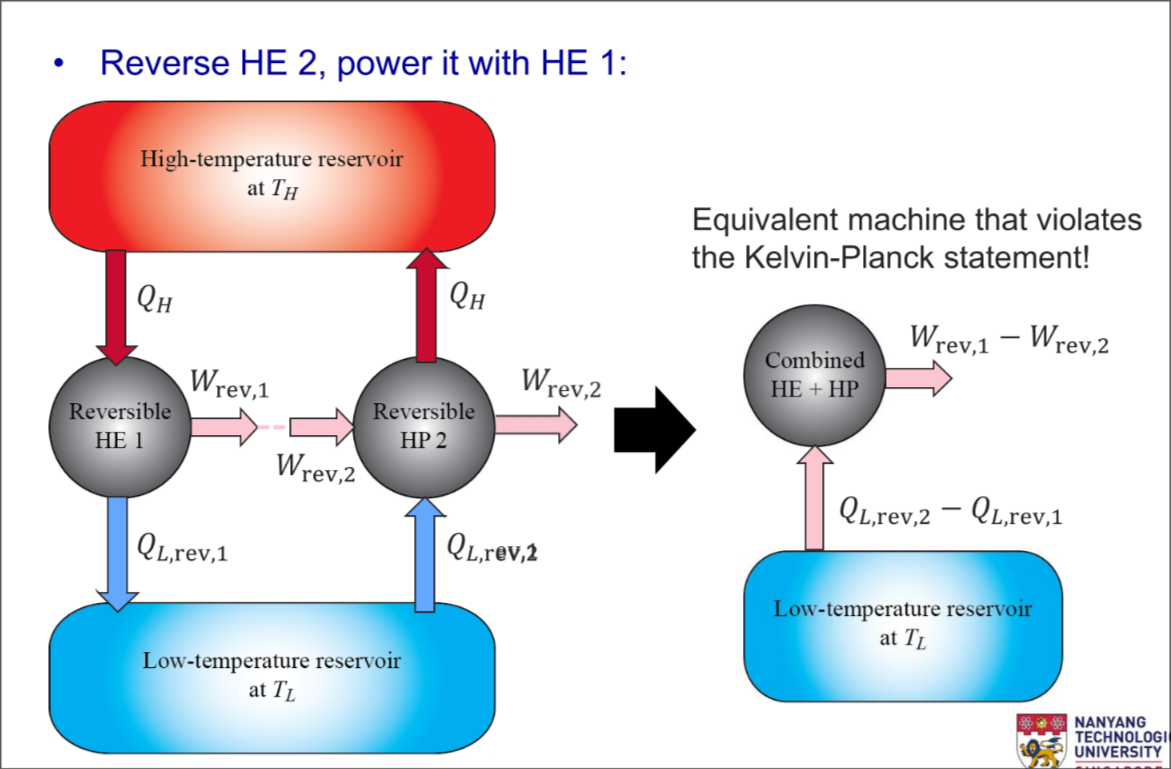
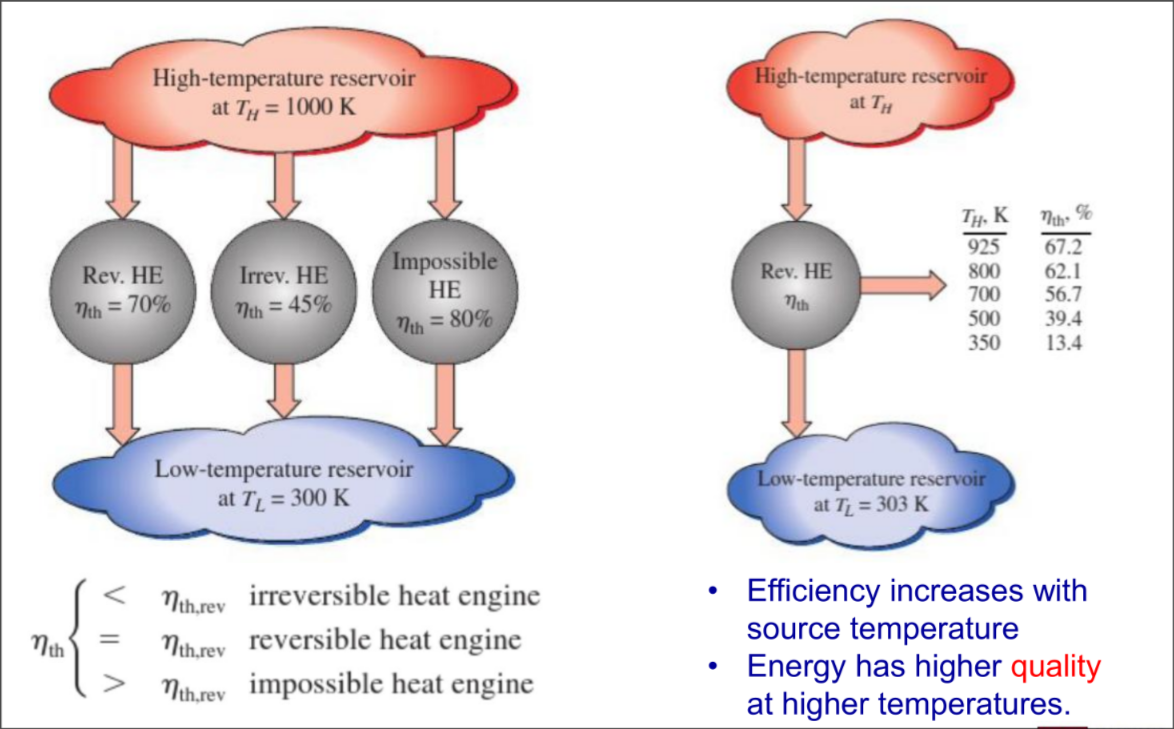
Carnot Principles
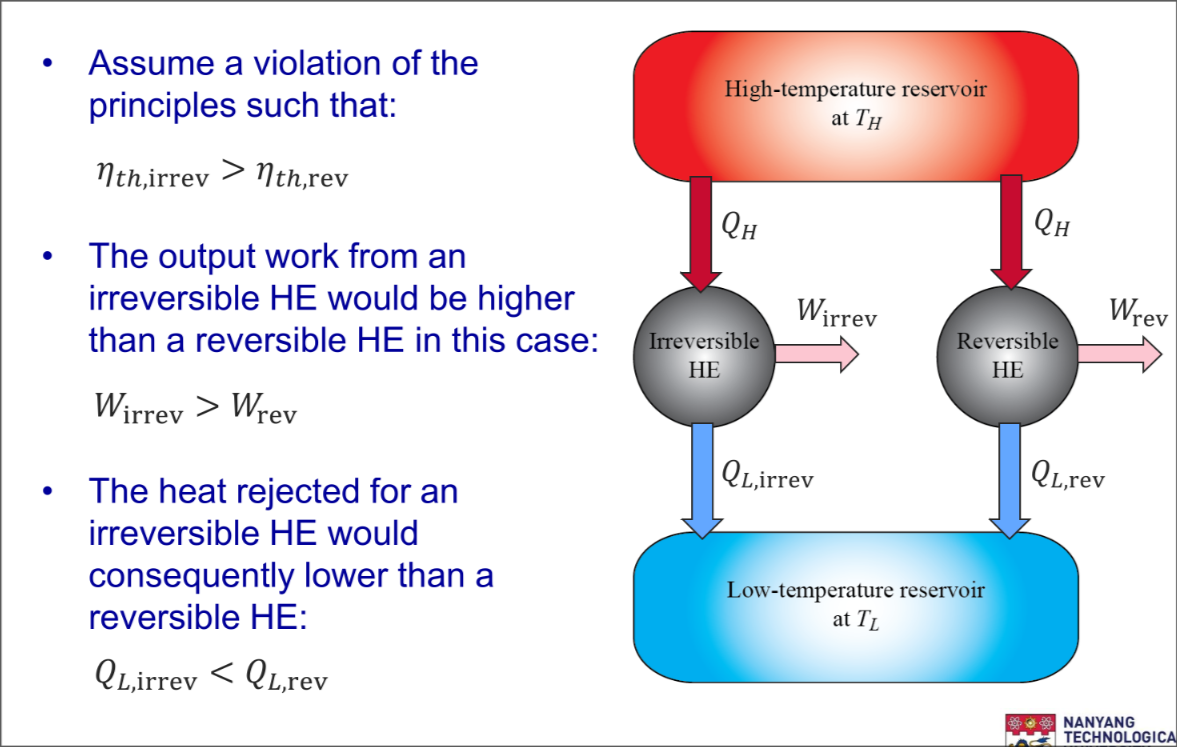
- The efficiency of an irreversible heat engine is always less than the efficiency of a reversible one operating between the same two reservoirs
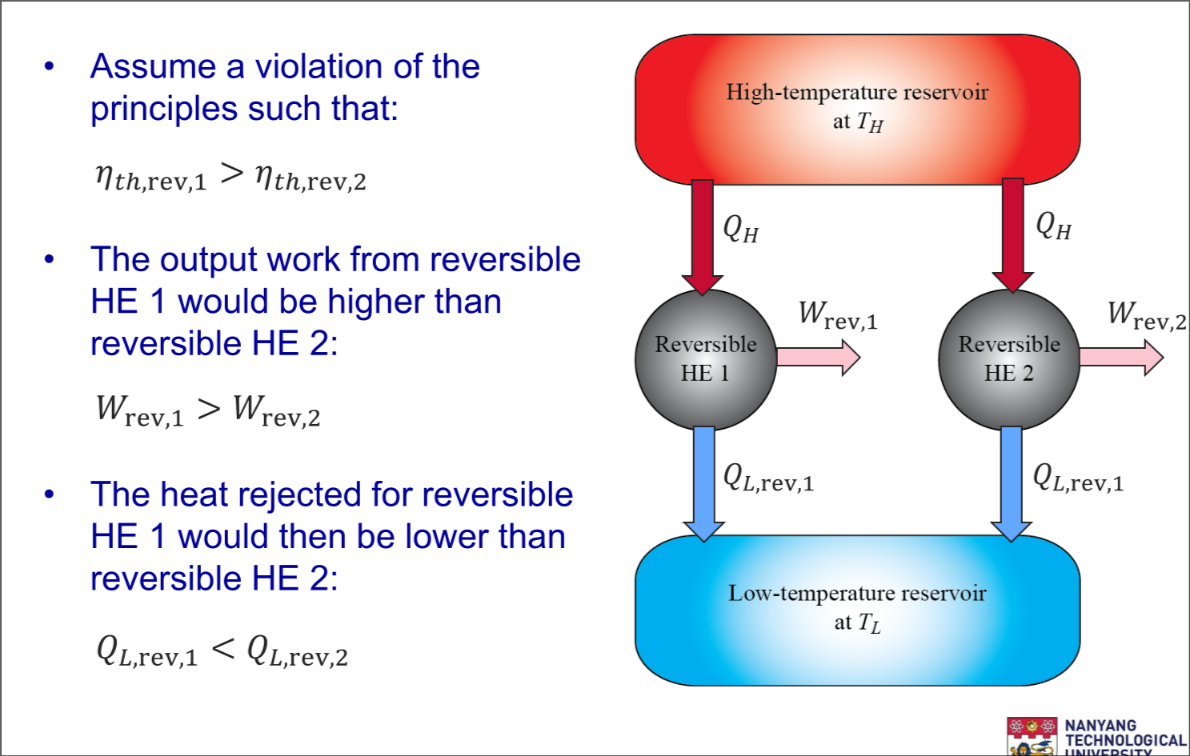
- The efficiencies of all reversible heat engines operating between the same two reservoirs are the same
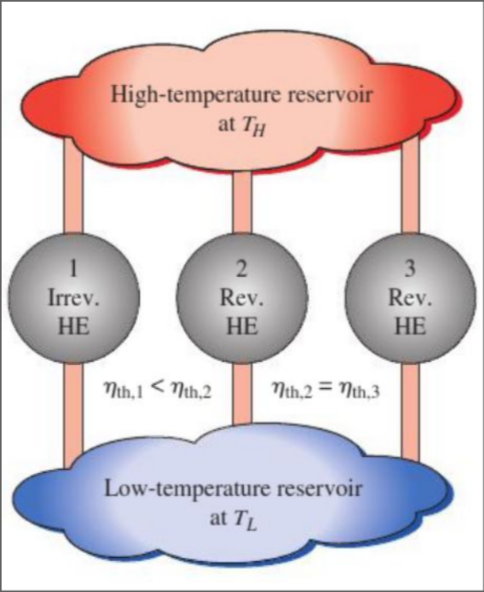
Expand for Proof of Carnot Principles
Part 1
Part 2
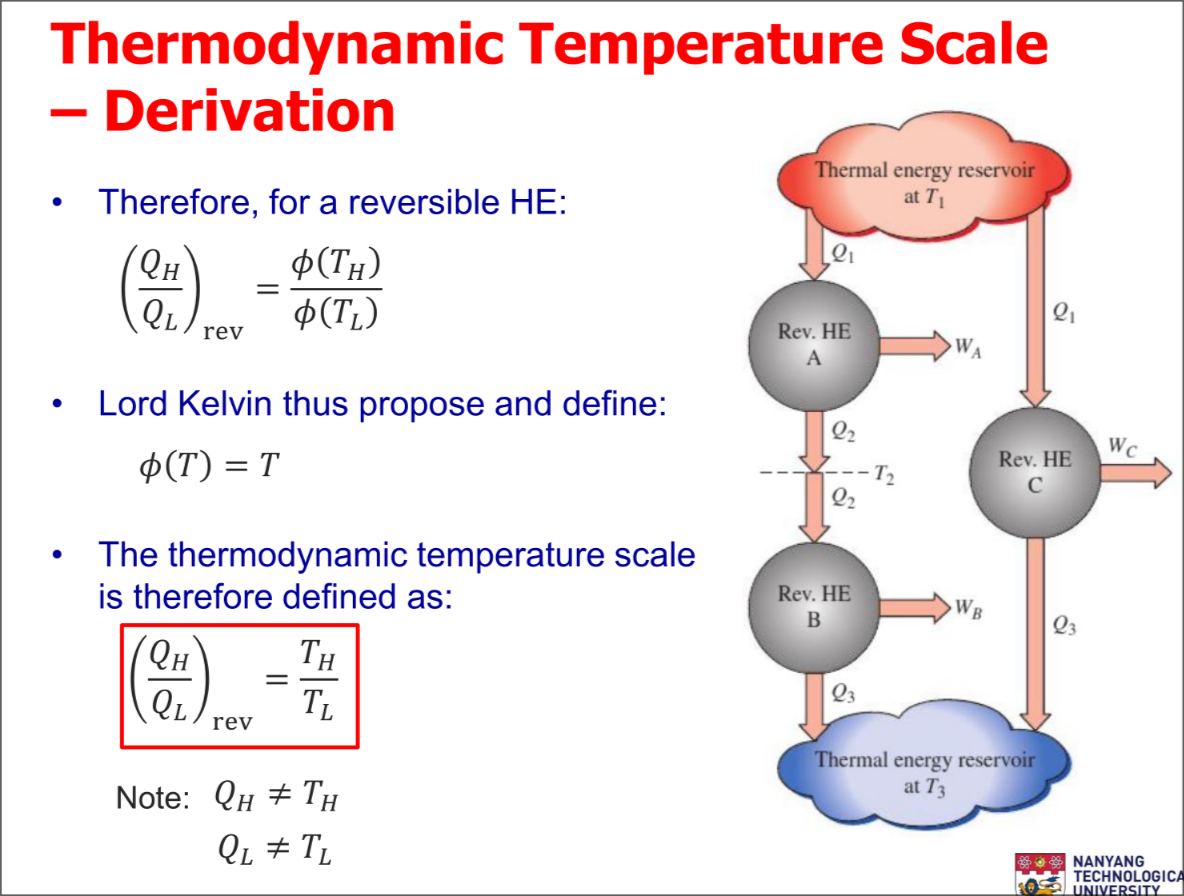
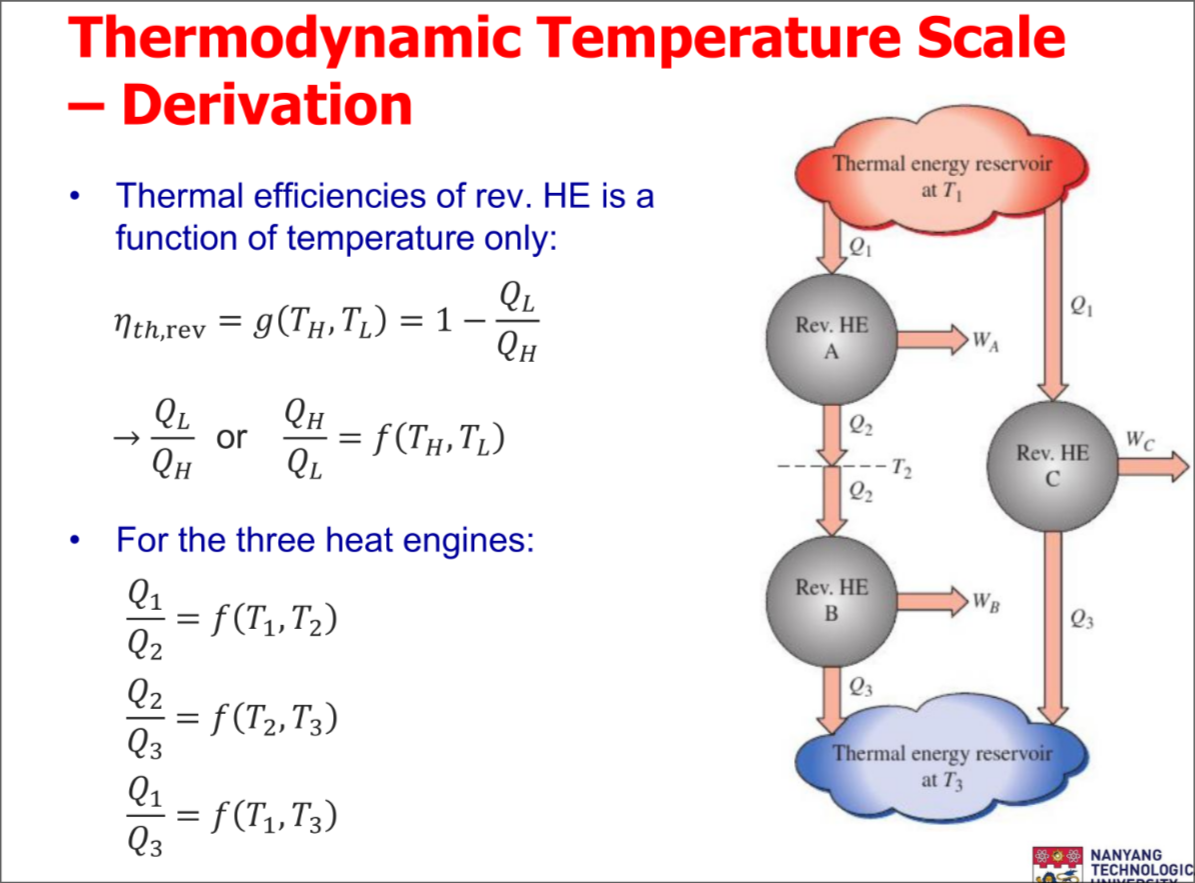
Thermodynamic Temperature Scale
A temperature scale that is independent of the properties of substances that are used to measure temperature is called a thermodynamic temperature scale
- Thermal reservoirs are characterized only by their temperatures
Derivation of the scale
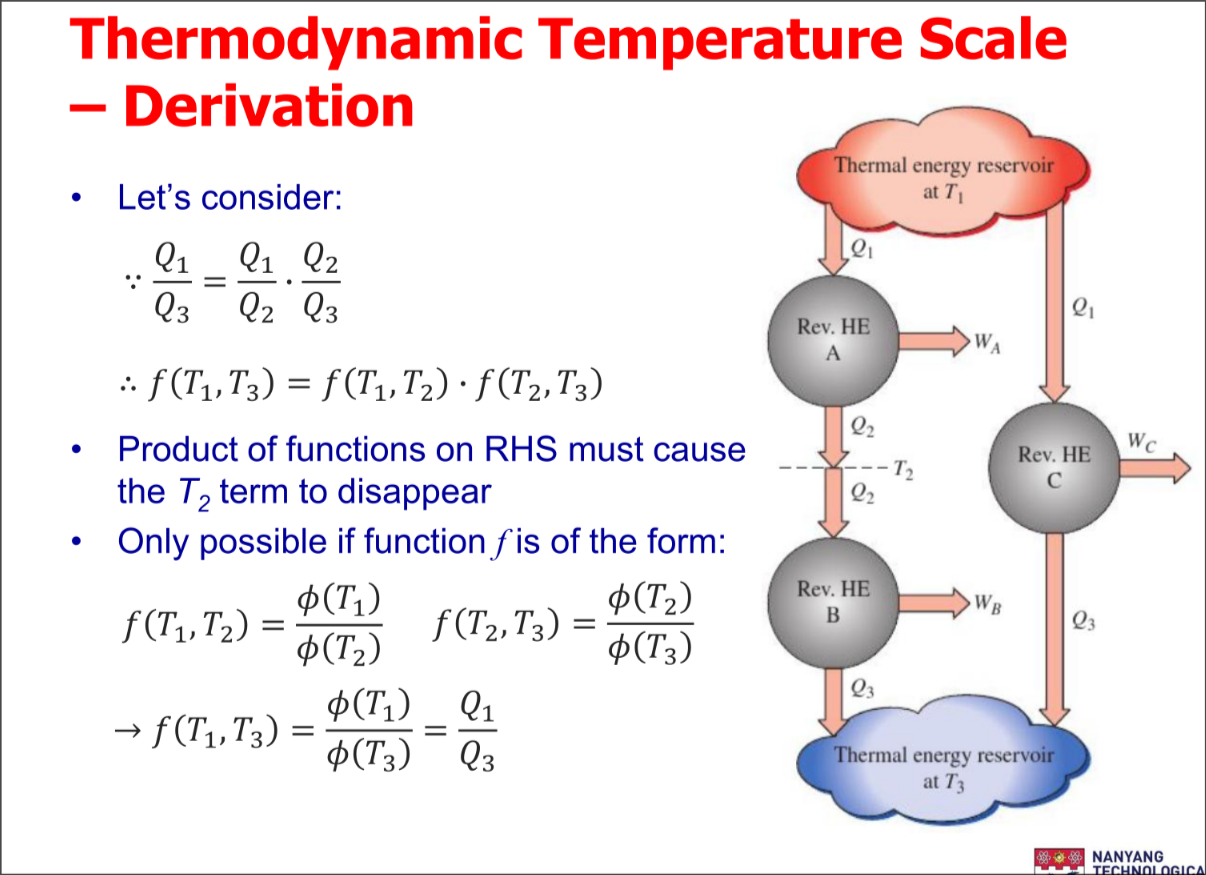
Carnot devices
AKA ideal / most efficient devices based on carnot principles
Carnot heat engine
Thermal efficiency of a Carnot heat engine
!! absolute temps only !!
Carnot refrigerator & heat pump
Coefficient of performance for a Carnot refrigerator:
Coefficient of performance for a Carnot heat pump: